Management of inflammatory bowel disease with oral serum-derived bovine immunoglobulin
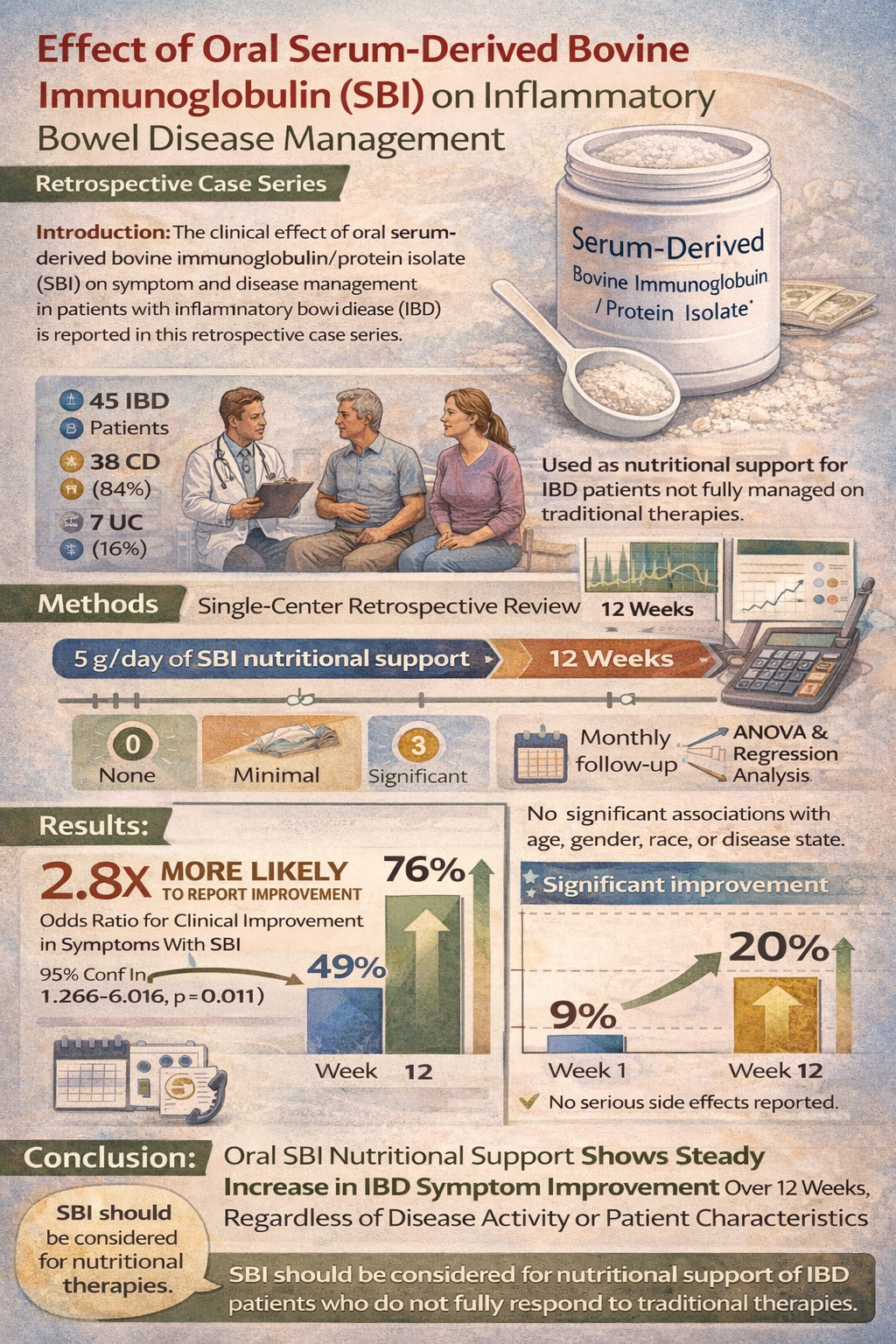
Abstract Introduction: The clinical effect of oral serum-derived bovine immunoglobulin/protein isolate (SBI) on symptom and disease management in patients with inflammatory bowel disease (IBD) is reported in this retrospective case series. Methods: A single-center, retrospective chart review of IBD patients [N = 45; Crohn’s disease (CD), n = 38 and ulcerative colitis (UC), n = 7] with limited to no response to traditional pharmaceutical therapies in controlling symptoms was performed after providing SBI (5 g/day) for nutritional support. Patients were contacted at least monthly to assess response to SBI for symptom management measured by a Likert scale (0 = none; 1 = minimal; 2 = moderate; 3 = significant; 4 = complete). Analysis of variance (ANOVA) was performed on response to therapy based on patient characteristics (age, gender, race) and IBD diagnosis. A multivariate ordered logistical regression model was performed to determine the odds ratio in overall disease management between week 1 and week 12. Finally, the overall group response and percent improvement to SBI was determined over 12 weeks. Results: The odds ratio from the regression model demonstrated that IBD patients were 2.8 times more likely to report clinical improvement in symptom scores with the addition of SBI to their therapeutic regimens [95% confidence interval (CI) 1.266–6.016, p = 0.011]. Disease management was not significantly associated with age, gender, race or disease state. The percentage of patients reporting a response to SBI therapy at week 1 was 49% which increased to 76% after 12 weeks with the fraction of responders gaining significant symptom improvement doubling during the same time period (9% versus 20%). Overall, this group of IBD patients showed increased, steady response to SBI therapy between week 1 and 12 with no reported side effects. Conclusion: These results suggest that SBI improves clinical management of IBD patients who are not fully managed on traditional therapies. SBI should be considered for the nutritional support of IBD regardless of disease activity, location, phenotype, duration, or complexity.
Serum-derived bovine immunoglobulin/protein isolate in the alleviation of chemotherapy-induced mucositis
Abstract
Background
Gastrointestinal (GI) mucositis caused by chemotherapy is associated with diarrhoea and intestinal barrier disruption caused by apoptosis, immune dysfunction and microbiome alterations. Serum-derived bovine immunoglobulin/protein isolate (SBI) has been shown to manage HIV-associated enteropathy and irritable bowel syndrome with diarrhoea (IBS-D). We investigated in a rat model whether SBI was effective in alleviating symptoms of irinotecan-induced GI mucositis.
Methods
Animals were gavaged with 250 or 500 mg/kg of SBI twice daily for 4 days, before intraperitoneal administration of 200 mg/kg irinotecan. Twice daily gavaging of SBI continued for 6 days post-irinotecan. Animals were monitored for bodyweight changes and incidence of diarrhoea and clinical symptoms of stress. Tissues and blood samples were collected at necropsy 6 h, and 2, 4 and 6 days post-irinotecan. H&E-stained colon and jejunum were analysed for histological damage.
Results
The overall incidence, severity and duration of diarrhoea, and clinical symptoms of mucositis were decreased in irinotecan-treated animals that had received SBI. Animals receiving 500 mg/kg SBI also tended to lose less bodyweight than animals treated only with irinotecan (P > 0.10). SBI-gavaged animals had less pronounced irinotecan-induced changes in neutrophil (P = 0.04959) and lymphocyte (P = 0.0035) levels, and lower tissue damage scores than those receiving irinotecan alone (P < 0.0001).
Conclusions
Twice daily oral gavage of SBI was well-tolerated and reduced the incidence, severity and duration of irinotecan-induced mucositis. SBI was associated with less pronounced changes in inflammatory cell levels and tissue damage to colon and jejunum. Ongoing experiments aim to investigate the mechanisms of SBI-associated gastrointestinal protection.